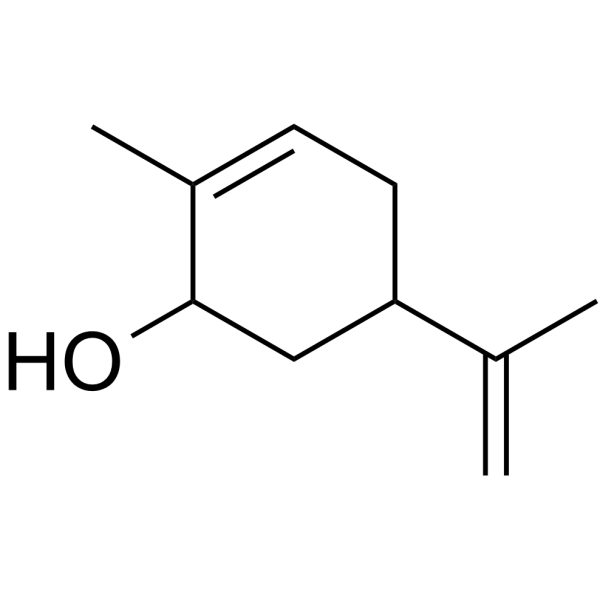
Carveol
CAS No. 99-48-9
Carveol( —— )
Catalog No. M29289 CAS No. 99-48-9
(-)-Carveol, mixture of isomers is a monocyclic monoterpenic alcohol, present in essential oils of plant species such as Cymbopogon giganteus, Illicium pachyphyllum and in spices such as Carum carvi (cumin).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 500MG | 38 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameCarveol
-
NoteResearch use only, not for human use.
-
Brief Description(-)-Carveol, mixture of isomers is a monocyclic monoterpenic alcohol, present in essential oils of plant species such as Cymbopogon giganteus, Illicium pachyphyllum and in spices such as Carum carvi (cumin).
-
Description(-)-Carveol, mixture of isomers is a monocyclic monoterpenic alcohol, present in essential oils of plant species such as Cymbopogon giganteus, Illicium pachyphyllum and in spices such as Carum carvi (cumin).(In Vitro):(-)-Carveol exhibited a significant vasorelaxant effect on KCl and 5-HT-induced contractions, obtaining EC50 values of 344.25 ± 8.4 and 175.82 ± 4.05 μM, respectively. The participation of calcium channels in the relaxation produced by (-)-carveol was analyzed using vessels pre-incubated with (-)-carveol (2000 μM) in a calcium-free medium, where the induction of contractions was abolished. The vasorelaxant effect of (-)-carveol on HUAs was reduced by tetraethylammonium (TEA), which increased the (-)-carveol EC50 to 484.87 ± 6.55 μM. The present study revealed that (-)-carveol possesses a vasorelaxant activity in HUAs, which was dependent on the opening of calcium and potassium channels.(In Vivo):(-)-Carveol has low toxicity, with a lethal dose 50% (LD50) equal to or greater than 2,500 mg/kg according to OECD guide no 423. In all gastric ulcer induction methods evaluated, (-)-Carveol (25, 50, 100 and 200 mg/kg, p.o.) significantly reduced the ulcerative lesion in comparison with the respective control groups. In the experimental protocol of pylorus ligation-induced gastric ulcer, (-)-Carveol (100 mg/kg) reduced (p < 0.001) the volume of gastric secretion in both routes (oral and intraduodenal). The previous administration of blockers NEM (sulfhydryl groups blocker), L-NAME (nitric oxide synthesis inhibitor), glibenclamide (KATP channel blocker) and indomethacin (cyclo-oxygenase inhibitor), significantly reduced the gastroprotection exercised by (-)-Carveol, suggesting the participation of these pathways in its gastroprotective activity. In addition, treatment with (-)-Carveol (100 mg/kg) increased (p < 0.001) mucus adhered to the gastric wall. Treatment also increased (p < 0.001) levels of reduced glutathione (GSH), superoxide dismutase (SOD) and interleukin-10 (IL-10). It also reduced (p < 0.001) malondialdehyde (MDA), myeloperoxidase (MPO), interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) levels.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayProteasome/Ubiquitin
-
TargetEndogenous Metabolite
-
RecptorEndogenous Metabolite
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number99-48-9
-
Formula Weight152.23
-
Molecular FormulaC10H16O
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (656.90 mM)
-
SMILESCC(C(C1)CC=C(C)C1O)=C
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Elaidic acid
Elaidic acid is the 9-trans isomer of oleic acid. It is a monounsaturated trans-fatty acid which can be found in partially hydrogenated cooking oils.
-
3-Hydroxyglutaric ac...
3-Hydroxyglutaric acid is one of several metabolites produced when insufficient levels of GCDH are available. It is used as a biomarker of GCDH deficiency.
-
D-Mannose
D-Mannose is a carbohydrate which plays an important role in human metabolism especially in the glycosylation of specific proteins.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


