AZD-7325
CAS No. 942437-37-8
AZD-7325( AZD7325 )
Catalog No. M16745 CAS No. 942437-37-8
AZD-7325 is a potent, α2/3 functionally selective, partial GABAA receptor modulator with pKi of 9.51.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 67 | In Stock |


|
| 5MG | 61 | In Stock |


|
| 10MG | 92 | In Stock |


|
| 25MG | 166 | In Stock |


|
| 50MG | 276 | In Stock |


|
| 100MG | 416 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameAZD-7325
-
NoteResearch use only, not for human use.
-
Brief DescriptionAZD-7325 is a potent, α2/3 functionally selective, partial GABAA receptor modulator with pKi of 9.51.
-
DescriptionAZD-7325 is a potent, α2/3 functionally selective, partial GABAA receptor modulator with pKi of 9.51; exerts neutral antagonism at the α1-subunit and partial efficacy at the α2,3-subunits over the α5-subunit; AZD7325 is expected to reduce the risk for the benzodiazepine like side effects, such as sedation and cognitive effects, and shows potential for the treatment of anxiety.Anxiety Phase 1 Clinical(In Vitro):AZD7325 is a high affinity and selective modulator of the GABAA receptor system, exhibits high binding affinity at GABAAα1, α2 and α3 (Ki=0.5, 0.3, and 1.3 nM, respectively), and low at GABAAα5 (Ki=230 nM).AZD7325 (0-10 μM; 3 consecutive days; once daily) causes a maximal CYP1A2 mRNA expression of 3.2-fold, 2.1-fold, and 2.5-fold in human hepatocytes from donor HH210, HH215, and HH216, respectively.AZD7325 (0-10 μM; 3 consecutive days; once daily) causes CYP1A2 and CYP3A4 protein expression in human hepatocytes from donor HH210.(In Vivo):AZD7325 (oral administration; 10, 17.8 or 31.6 mg/kg; 30 minutes before the induction of hyperthermia) attenuates hyperthermia-induced seizures, shows median thresholds in the treatment groups of 42.8°C for 10 mg/kg, 43.3°C for 17.8 mg/kg, and 43.4°C for 31.6 mg/kg compares to 42.2°C in vehicle group.
-
In VitroRT-PCRCell Line:Primary human hepatocytes from one female (HH210) and two male (HH215, HH216) donorsConcentration:0.01, 0.1, 1, 10 μM Incubation Time:3 consecutive days Result:Led to increase of CYP1A2 mRNA expression.Western Blot Analysis Cell Line:Primary human hepatocytes from donors Concentration:0.01, 0.1, 1, 10 μM Incubation Time:3 consecutive days Result:Increased CYP1A2 and CYP3A4 protein level.
-
In VivoAnimal Model:Male and female P18 - P20 F1.Scn1a+/- mice Dosage:10, 17.8 or 31.6 mg/kg Administration:Oral administration; 30 minutes before the induction of hyperthermia Result:Attenuated hyperthermia-induced seizures in F1.Scn1a+/- mice with no sedative effect.
-
SynonymsAZD7325
-
PathwayMembrane Transporter/Ion Channel
-
TargetGAT
-
RecptorGAT
-
Research AreaNeurological Disease
-
IndicationAnxiety
Chemical Information
-
CAS Number942437-37-8
-
Formula Weight354.385
-
Molecular FormulaC19H19FN4O2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (282.18 mM)
-
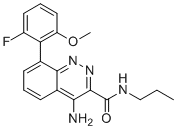
SMILESO=C(C1=NN=C2C(C3=C(OC)C=CC=C3F)=CC=CC2=C1N)NCCC
-
Chemical Name4-amino-8-(2-fluoro-6-methoxyphenyl)-N-propylcinnoline-3-carboxamide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Chen X, et al. Br J Clin Pharmacol. 2014 Dec;78(6):1298-314.
2. Zhou D, et al. Br J Clin Pharmacol. 2012 Jul;74(1):98-108.
3. Jucaite A, et al. Psychopharmacology (Berl). 2017 Feb;234(4):707-716.
4. Alhambra C, et al. Bioorg Med Chem. 2011 May 1;19(9):2927-38.
molnova catalog



related products
-
KRM-II-81
KRM-II-81 is a potent, selective α1/α2/α3 GABAA receptors positive allosteric modulator with EC50 of 937 nM for α1β3γ2, negligible efficacy at α4/α5/α6 GABAA receptors.
-
TP003
TP003 is a potent, functional selectivity for α3 subunit-containing GABAA receptor agonist with Ki of <1 nM for α1β3γ2, α2β3γ2, α3β3γ2 and α5β3γ2.
-
DCBS152A
DCBS152A is a potent, functionally selectiver negative modulator of GABAA receptor at the modulatory PQ site in some receptor isoforms.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


