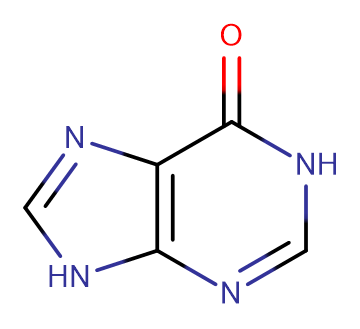
6-Hydroxypurine
CAS No. 68-94-0
6-Hydroxypurine( 6-Hydroxypurine | NSC 14665 | NSC 129419 )
Catalog No. M15636 CAS No. 68-94-0
A purine and a reaction intermediate in the metabolism of adenosine and in the formation of nucleic acids by the salvage pathway.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | 45 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name6-Hydroxypurine
-
NoteResearch use only, not for human use.
-
Brief DescriptionA purine and a reaction intermediate in the metabolism of adenosine and in the formation of nucleic acids by the salvage pathway.
-
DescriptionA purine and a reaction intermediate in the metabolism of adenosine and in the formation of nucleic acids by the salvage pathway.
-
In Vitro——
-
In Vivo——
-
Synonyms6-Hydroxypurine | NSC 14665 | NSC 129419
-
PathwayOthers
-
TargetOther Targets
-
RecptorPurine nucleoside phosphorylase
-
Research AreaOther Indications
-
Indication——
Chemical Information
-
CAS Number68-94-0
-
Formula Weight136.11
-
Molecular FormulaC5H4N4O
-
Purity>98% (HPLC)
-
SolubilityDMSO: 0.4 mg/mL (2.93 mM)
-
SMILESO=C1NC=NC2=C1N=CN2
-
Chemical Name1,9-dihydro-6H-purin-6-one
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Overington JP, et al. Nat Rev Drug Discov. 2006 Dec;5(12):993-6.
molnova catalog



related products
-
Erythrosin B
Iodeosin is a tetraiodofluorescein used as a red coloring in some foods (cherries, fish), as a disclosure of DENTAL PLAQUE, and as a stain of some cell types.
-
Dipsacus saponin X
The herbs of Dipsacus asper.
-
S6-1
S6 peptide is a potentially important lamin kinase. S6 peptide is involved in the process of cardiac hypertrophy induced by mechanical loading. S6 peptide can be activated by many kinds of growth factors.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


