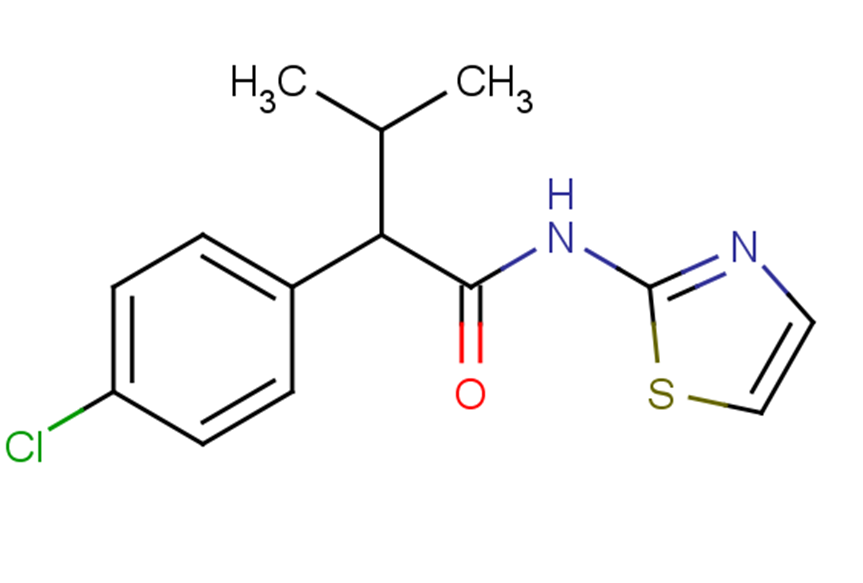
4-CMTB
CAS No. 300851-67-6
4-CMTB( —— )
Catalog No. M22376 CAS No. 300851-67-6
4-CMTB is a selective agonist of FFA2 and GPR43.It also is a positive allosteric modulator (pEC50=6.38).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 53 | In Stock |


|
| 5MG | 79 | In Stock |


|
| 10MG | 126 | In Stock |


|
| 25MG | 219 | In Stock |


|
| 50MG | 323 | In Stock |


|
| 100MG | 480 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name4-CMTB
-
NoteResearch use only, not for human use.
-
Brief Description4-CMTB is a selective agonist of FFA2 and GPR43.It also is a positive allosteric modulator (pEC50=6.38).
-
Description4-CMTB is a selective agonist of FFA2 and GPR43.It also is a positive allosteric modulator (pEC50=6.38).
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
Recptorfree fatty acid receptor 2 (FFA2/GPR43)
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number300851-67-6
-
Formula Weight294.8
-
Molecular FormulaC14H15ClN2OS
-
Purity>98% (HPLC)
-
SolubilityDMSO:100 mg/mL (339.21 mM; Need ultrasonic)
-
SMILESCC(C)C(C(=O)Nc1nccs1)c1ccc(Cl)cc1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Milligan G, Stoddart LA, Smith NJ. Agonism and allosterism: the pharmacology of the free fatty acid receptors FFA2 and FFA3. Br J Pharmacol. 2009;158(1):146-153.
molnova catalog



related products
-
Human IgG1 Control
Human IgG1 Control was purified from Human serum by chromatography.Human IgG1 Control can be used as a Control, standard, blocking agent, or coating protein in a variety of experiments, including ELISA, Western and blotting, immunoprecipitation, and immunoelectrophoresis.Human IgG1 Control can also be used as an antigen or ligand in immunochemical conjugate reactions.
-
Intermedine
(+)-Intermedine, a pyrrolizidine alkaloid (PA), shows remarkable cytotoxic activity against neural progenitor cells (NPCs).
-
p-Phenylenediamine, ...
p-Phenylenediamine, N,N'-diphenyl- is a bioactive chemical. It has been used to prevent vitamin E deficiency in lambs.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


