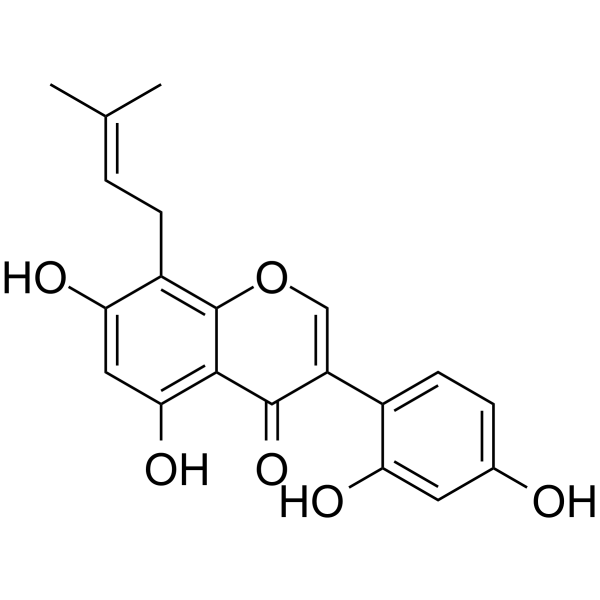
2,3-Dehydrokievitone
CAS No. 74161-25-4
2,3-Dehydrokievitone( —— )
Catalog No. M31058 CAS No. 74161-25-4
2,3- Dehydrokievitone exhibits strong cytotoxic activity and induces apoptosis efficiently in cancer cells.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 231 | In Stock |


|
| 50MG | Get Quote | In Stock |


|
| 100MG | Get Quote | In Stock |


|
Biological Information
-
Product Name2,3-Dehydrokievitone
-
NoteResearch use only, not for human use.
-
Brief Description2,3- Dehydrokievitone exhibits strong cytotoxic activity and induces apoptosis efficiently in cancer cells.
-
Description2,3- Dehydrokievitone exhibits strong cytotoxic activity and induces apoptosis efficiently in cancer cells.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number74161-25-4
-
Formula Weight354.4
-
Molecular FormulaC20H18O6
-
Purity>98% (HPLC)
-
Solubility——
-
SMILES——
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Super-TDU (TFA) (159...
uper-TDU is an inhibitory peptide targeting YAP-TEADs interaction.
-
3'-O-Angeloylhamaudo...
3'-O-Angeloylhamaudol has anti-inflammatory activity. 3'-O-Angeloylhamaudol induces apoptosis through the mitochondial-dependent apoptotic pathway.
-
W-2429
W-2429 (NSC294836) is a non-narcotic analgesic that may be used in the study of neurological disorders.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


