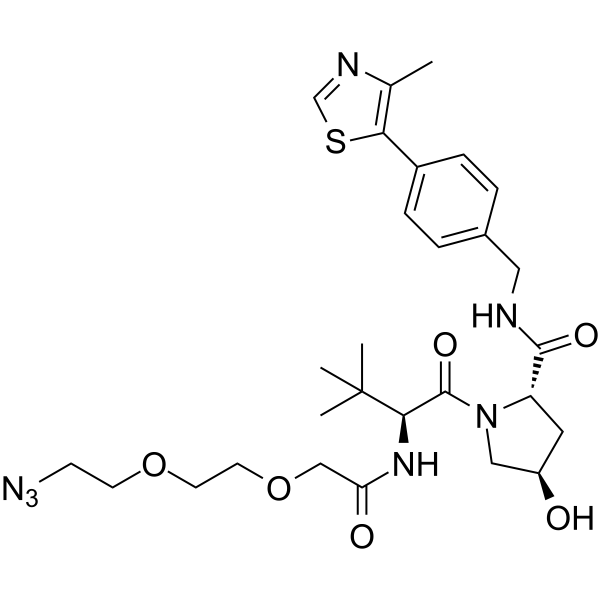
(S,R,S)-AHPC-PEG2-N3
CAS No. 2010159-45-0
(S,R,S)-AHPC-PEG2-N3( VH032-PEG2-N3 | VHL Ligand-Linker Conjugates 6 | E3 ligase Ligand-Linker Conjugates 13 )
Catalog No. M26945 CAS No. 2010159-45-0
(S,R,S)-AHPC-PEG2-N3 is a synthesized E3 ligase ligand-linker conjugate containing a (S,R,S)-AHPC based VHL ligand and 2 unit PEG linker.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 81 | Get Quote |


|
| 10MG | 115 | Get Quote |


|
| 25MG | 210 | Get Quote |


|
| 50MG | 302 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product Name(S,R,S)-AHPC-PEG2-N3
-
NoteResearch use only, not for human use.
-
Brief Description(S,R,S)-AHPC-PEG2-N3 is a synthesized E3 ligase ligand-linker conjugate containing a (S,R,S)-AHPC based VHL ligand and 2 unit PEG linker.
-
Description(S,R,S)-AHPC-PEG2-N3 is a synthesized E3 ligase ligand-linker conjugate containing a (S,R,S)-AHPC based VHL ligand and 2 unit PEG linker.
-
In Vitro——
-
In Vivo——
-
SynonymsVH032-PEG2-N3 | VHL Ligand-Linker Conjugates 6 | E3 ligase Ligand-Linker Conjugates 13
-
PathwayOthers
-
TargetOther Targets
-
RecptorCalcium Channel
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number2010159-45-0
-
Formula Weight601.72
-
Molecular FormulaC28H39N7O6S
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 125 mg/mL (207.74 mM)
-
SMILESO=C(N[C@@H](C(C)(C)C)C(N(C[C@H](O)C1)[C@@H]1C(NCC2=CC=C(C(SC=N3)=C3C)C=C2)=O)=O)COCCOCCN=[N+]=[N-]
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Vega JA, et al. Effect of treatment with the dihydropyridine-type calcium antagonist darodipine (PY 108-068) on the expression of neurofilament protein immunoreactivity in the cerebellar cortex of aged rats. Mech Ageing Dev. 1994 Aug;75(2):169-77.
molnova catalog



related products
-
Propargyl-PEG11-meth...
Propargyl-PEG11-methane is a PEG-based PROTAC linker that can be used to synthesize PROTACs.
-
Adenine hydrochlorid...
Adenine HCl is a hydrochloride salt form of adenine which is a nucleobase with a variety of roles in biochemistry.
-
(+)-alpha-Pinene
(1R)-α-Pinene is a natural product for research related to life sciences.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


