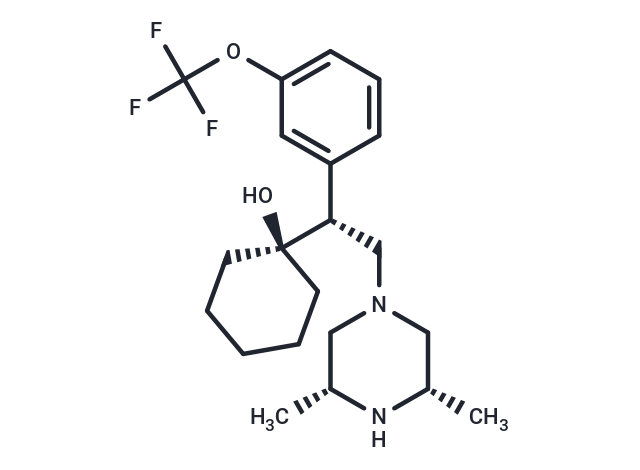
(Iso)-WAY-260022
CAS No. 1214914-38-1
(Iso)-WAY-260022( —— )
Catalog No. M37523 CAS No. 1214914-38-1
(Iso)-WAY-260022 ((Iso)-NRI-022) is a stereoisomer of WAY-260022, a norepinephrine reuptake inhibitor and selective inhibitor of 5-hydroxytryptamine and dopamine transport proteins.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 296 | Get Quote |


|
| 5MG | 459 | Get Quote |


|
| 10MG | 657 | Get Quote |


|
| 25MG | 994 | Get Quote |


|
| 50MG | 1371 | Get Quote |


|
| 100MG | 1773 | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product Name(Iso)-WAY-260022
-
NoteResearch use only, not for human use.
-
Brief Description(Iso)-WAY-260022 ((Iso)-NRI-022) is a stereoisomer of WAY-260022, a norepinephrine reuptake inhibitor and selective inhibitor of 5-hydroxytryptamine and dopamine transport proteins.
-
Description(Iso)-WAY-260022 ((Iso)-NRI-022) is a stereoisomer of WAY-260022, a norepinephrine reuptake inhibitor and selective inhibitor of 5-hydroxytryptamine and dopamine transport proteins.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1214914-38-1
-
Formula Weight400.48
-
Molecular FormulaC21H31F3N2O2
-
Purity>98% (HPLC)
-
Solubility——
-
SMILES[C@@H](CN1C[C@H](C)N[C@H](C)C1)([C@]2(O)CCCCC2)C3=CC(OC(F)(F)F)=CC=C3
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Matairesinol
Matairesinol has radical and superoxide scavenging activities; it also has anti-angiogenic activity by suppressing mROS signaling , can decrease hypoxia-inducible factor-1α± in hypoxic HeLa cells.
-
Opicinumab
Opicinumab (BIIB033) is a novel monoclonal antibody against LINGO-1 that may be used to prevent and delay acute optic neuritis and recurrent multiple sclerosis.
-
I-BET567
I-BET567, a potent pan-BET inhibitor with oral activity, demonstrates pIC50 values of 6.9 and 7.2 for BRD4 BD1 and BD2, respectively. This compound has exhibited efficacy in mouse models of oncology and inflammation .



 Cart
Cart
 sales@molnova.com
sales@molnova.com


