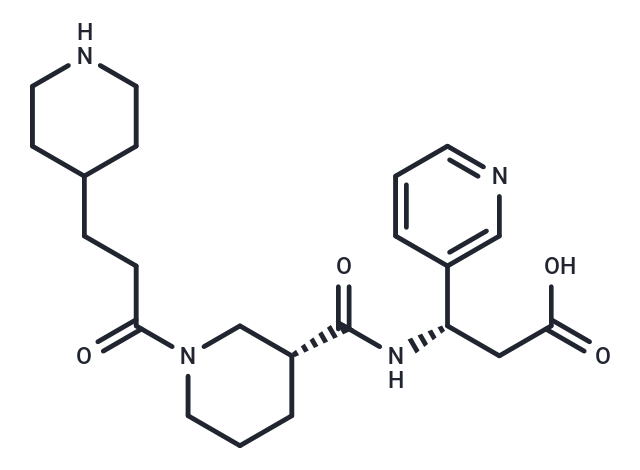
Elarofiban
CAS No. 198958-88-2
Elarofiban( —— )
Catalog No. M34233 CAS No. 198958-88-2
Elarofiban(RWJ-53308) is a novel and orally active GPIIb/IIIa antagonist.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 603 | Get Quote |


|
| 5MG | 922 | Get Quote |


|
| 10MG | 1226 | Get Quote |


|
| 25MG | 1794 | Get Quote |


|
| 50MG | 2410 | Get Quote |


|
| 100MG | 3177 | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameElarofiban
-
NoteResearch use only, not for human use.
-
Brief DescriptionElarofiban(RWJ-53308) is a novel and orally active GPIIb/IIIa antagonist.
-
DescriptionElarofiban (RWJ-53308) is a nonpeptide, orally active fibrinogen receptor antagonist. Elarofiban has the potential for platelet mediated thrombotic disorders research.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayCell Cycle/DNA Damage
-
TargetIntegrin
-
RecptorIntegrin
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number198958-88-2
-
Formula Weight416.51
-
Molecular FormulaC22H32N4O4
-
Purity>98% (HPLC)
-
Solubility——
-
SMILES[C@H](NC(=O)[C@H]1CN(C(CCC2CCNCC2)=O)CCC1)(CC(O)=O)C=3C=CC=NC3
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Judith H. Cohen, et al. A Practical Synthesis of the Platelet Fibrinogen Antagonist, Elarofiban. Organic Process Research & Development 2003, 7, 866-872.
molnova catalog



related products
-
Tirofiban
A potent, selective inhibitor of the platelet fibrinogen receptor GPIIb/IIIa with IC50 of 9 nM.
-
RO0270608
RO0270608, the active metabolite of R411, is an orally active dual α4β1/α4β7 integrin antagonist with anti-inflammatory properties, useful for studying allergic inflammatory responses.
-
DS-70
DS-70 (α4 integrin inhibitor DS-70) is a novel potent α/β- peptidomimetic antagonist of α4 integrin.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


